11.1 REACTION RATE
11.1 REACTION RATE
1. Explain reaction rate, average rate,instantaneous rate and initial rate.
2. Explain the graph of concentration against
3. Write differential rate equation.
4. Determine the reaction rate base on a differential equation.
DEFINITION:
Chemical kinetics is the study of the rates of chemical reactions, the factors that affect these rates, and the reaction mechanisms by which reactions occur.
KEYWORD
- -Time
- -Optimum yield
- -Optimum conditions( Control over reaction, obtain products economically, using optimum conditions industrial process)
REACTION RATE
rate= -d[A]/dt d[A]=change in concentration of A
dt=period of time
rate= d[B]/dt d[B]=change in concentration of B
Because [A] decrease with time,d[A] is negative
Rate of Reaction
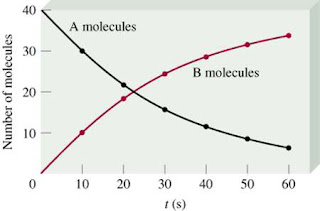
The average rate is the rate over a period of time.
Instantaneous rate is determined from a graph of concentration vs time by drawing a line tangent to the curve at that particular time.
o
The rate of reaction at a given time is called an instantaneous rate of reaction. o
The instantaneous rate at the beginning of a reaction is called the initial rate of reaction. o
The Differential Equation
A differential rate equation enables the relationship between the rate of disapearance of reactants and the formation of products.Consider the reaction.
Rate = -(1/a)(d[A]/dt) = -(1/b)(d[B]/dt) = (1/c)(d[C]/dt) = (1/d)(d[D]/dt)
o
Reaction:
Br2(aq) + HCOOH(aq) to 2Br(aq) + 2H(aq) + CO2
Instantaneous rate = rate for specific time
average rate = (-d[Br2]/dt = -[Br2]final – [Br2]initial)/(tfinal - tinitial)
Example:
equation for formation of NH3,
N2(g) + 3H2(g) to 2NH3(g)
The differential rate equation is:
rate= -d[N2]/dt = (-1/3)(d[H2]/dt) = (1/2)(d[NH3]/dt)
The equation means that the rate of dissapearence of N2 is 1/3 the rate of dissapearance of H2 and
1/2 the rate of formation of NH3.
- Reaction rate is the change in the concentration of a reactant or a product with time.
- Unit of rate is mole per liter per time (mol L-1s-1)
- rate directly proportional to 1/time